Abstract
Introduction: Disseminated intravascular coagulation (DIC) is an acquired syndrome characterized by widespread intravascular activation of coagulation complicating many conditions including sepsis and traumatic injuries. Early recognition and treatment of DIC is of paramount importance. However, to date no useful markers have been identified that can differentiate "pre-DIC" (which is destined to lead to DIC) from "without-DIC" (in which the hypercoagulable state is transient and does not lead to DIC). Activation of neutrophils by inflammatory stimuli or microbes results in the release of neutrophil extracellular traps (NETs). NETs are web-like structures consisting of cell-free DNA (cfDNA), histones, myeloperoxidase (MPO), and anti-microbial proteins. Although NETs aid in the host response to infection by sequestering pathogens, excessive production of NETs can exert collateral damage to the host by activating coagulation, inhibiting fibrinolysis, and causing endothelial cell death. Recently, sepsis-induced DIC has been shown to correlate with circulating levels of DNA-associated MPO, suggesting that the release of NETs by neutrophils plays a critical role in the onset of DIC. Our objective was to attempt to identify a mechanistic role for NETosis in the development DIC in sepsis and use this information to identify 'pre-DIC' signatures.
Methods: Clinical data and biological samples from 357 septic patients who were part of the DNA as a Prognostic Marker in ICU patient (DYNAMICS) study were used. Incidence of DIC was determined using the International Society on Thrombosis and Haemostasis (ISTH) scoring system on Day 1 and each subsequent day. We quantified levels of Citrullinated Histone H3 (H3Cit), a biomarker of NETosis, as well as levels of cfDNA. We also measured levels of Protein C (PC), a natural anticoagulant that prevents blood clotting in the microcirculation. Increased consumption of PC is a hallmark of sepsis and may lead to microvascular thrombosis and DIC.
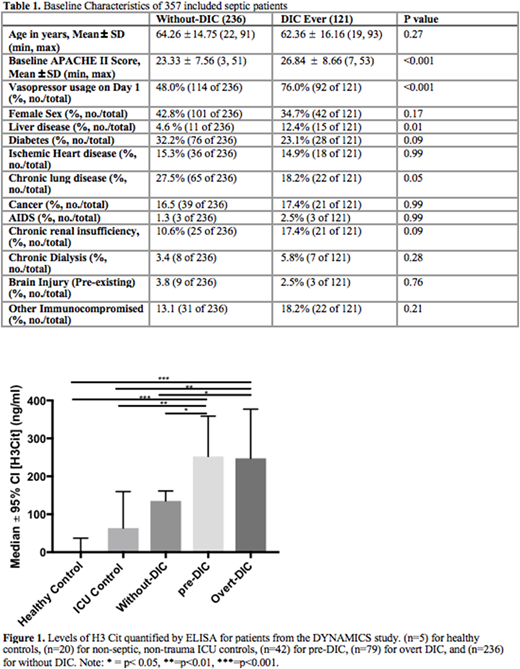
Results: Of the 357 patients included from the DYNAMICS study, 121 were classified as having DIC during the study period: 79 on Day 1 ('overt-DIC') and 42 on a subsequent day ('pre-DIC'). Baseline characteristics of patients are shown in Table 1. Those with DIC had significantly higher baseline APACHE II scores and were significantly more likely to be on vasopressors at admission or have a history of chronic liver disease. DIC was associated with significantly increased mortality (HR= 2.53; 95% CI = 1.62 - 3.93; p < 0.001) even when age and past medical history were controlled for. Levels of PC were significantly reduced in patients with DIC at all time points compared to those without DIC (p < 0.01). However, cfDNA levels did not differ between patients with and without DIC at any timepoint. As cfDNA may be released by multiple mechanisms and sources, H3 Cit was quantified on Day 1 as a marker of NETosis. Levels of H3 Cit on Day 1 were significantly higher in "pre-DIC" and "overt-DIC" patients compared to those 'without DIC' (p<0.05), non-septic, non-trauma ICU Controls (p<0.01), and healthy volunteers (p<0.001) (Figure 1). With respect to differentiating 'pre-DIC' from 'overt-DIC', using Day 1 H3 Cit provided an AUC of 0.66 (0.59-0.74). Higher H3 Cit levels were also correlated with lower PC levels in septic patients (r = -0.124; p = 0.02). In a comparison group of non-septic trauma patients also from the DYNAMICS study; (n=6) patients with DIC did not have significantly different Day 1 H3 Cit from (n=25) trauma patients without DIC (p=0.62) or ICU controls (p=0.99).
Conclusion: In sepsis, DIC pathophysiology reflects a consumptive process as indicated by reduced PC levels. NETosis may contribute to this process by producing pro-coagulant stimuli and may prove useful in identifying patients who will develop DIC. As a regulated and targetable process investigations involving NETosis may yield therapies for early treatment of DIC in sepsis.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal